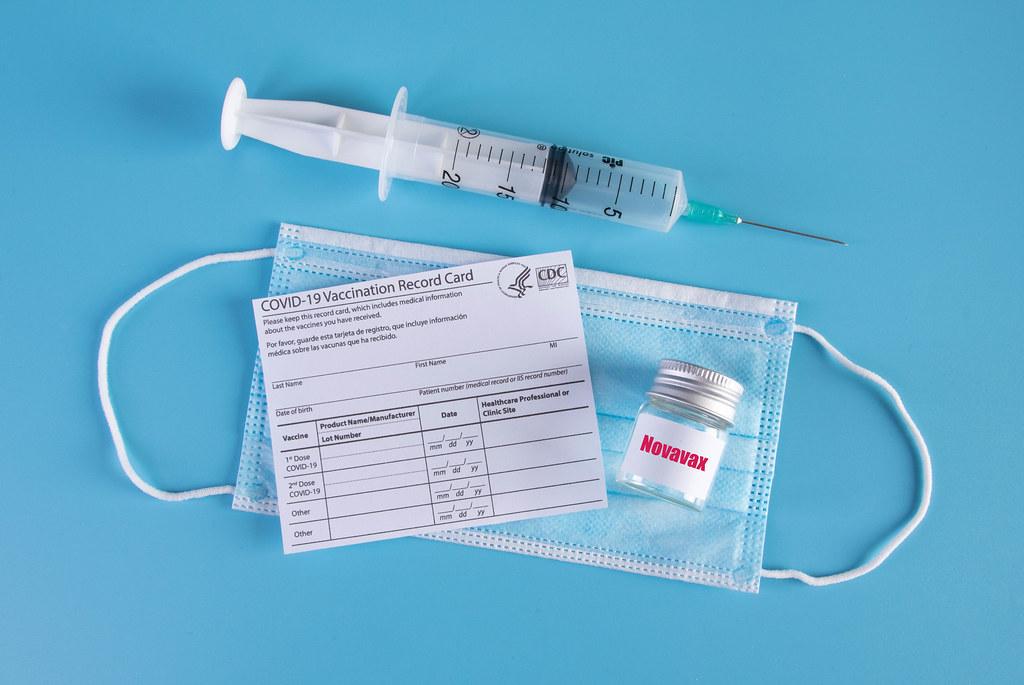
The pharmaceutical company, Pfizer, has just concluded a randomized control trial of its COVID vaccine on a key age group: children 5-12 years of age. The study, recently reviewed by the Food and Drug Administration (FDA) and published by the New England Journal of Medicine, demonstrated that the Pfizer BNT162b2 vaccine is highly safe and effective among children aged 5-12.
Just like the Pfizer vaccine for adults, the researchers determined that the best course of action is to administer two vaccine doses 21 days apart. However, a smaller dosage will be given to children aged 5-11 than what will be given to adults.
In the Pfizer trial for children, two-thirds of the 2,285 participants received the vaccine, while one-third received a saltwater placebo shot. The study only included children who have never had COVID-19 in the past. The results were consistent across genders, ethnicities, and racial groups. Data on side effects was collected starting with the administration of the first dose. COVID testing for the efficacy numbers of the vaccine was recorded starting a week after the second dose was administered in order to give the vaccine time to begin working.
The results of the trial look favorable for the Pfizer vaccine. There were three COVID cases in the vaccine group and 16 in the placebo group, despite there being over 700 more children in the vaccine group. The efficacy rate among children aged 5-11 was 90.7%, and zero severe COVID cases were recorded among either group, vaccine, or placebo. Vaccine efficacy is the percent reduction in positive cases versus the placebo group, so the 90.7% efficacy does not mean that 10% of vaccine recipients contracted COVID-19. Rather, it means that they were 90.7% less likely to get the virus than a child in the placebo group.
Any injection can cause side effects, but the Pfizer vaccine showed relatively low numbers of adverse events in vaccine recipients. The most common local adverse event was pain at the injection site. 74% of vaccine recipients experienced pain at the injection site after their first dose, and 71% reported this pain after the second injection. 31% of placebo recipients reported pain at the injection site after their first shot and 29% after the second dose. Thus, it can be concluded that the vaccine increases the likelihood of pain at the injection site.
Fatigue and headache were the most common systemic adverse events — meaning adverse events that do not occur near the injection site — among vaccine and placebo participants, but the rates of each event were about the same in both. This shows that the vaccine may not cause systemic adverse effects in any significant way. Essentially, local events in this trial were reported more among vaccine recipients, while systemic events were similar among both groups. 10.9% of vaccine recipients experienced some adverse event, versus 9.2% of placebo recipients, showing that the rate of increase in adverse events after receiving the Pfizer vaccine compared to receiving the placebo injection was just barely higher. Of course, pain at the injection site will be caused by any shot in some recipients, but it is promising that the Pfizer vaccine seems to elicit a minimal amount of increase in adverse events compared to the placebo.
Many people wonder why vaccinating children aged 5-11 against COVID-19 is even necessary, considering their lower rates of serious cases. However, many individuals with Multisystem inflammatory syndrome in children (MIS-C), a condition that causes inflammation of crucial organs, previously had COVID-19. This could be either correlation or causation, but experts believe it is causation. Additionally, as the deadlier Delta variant spreads across the world, and as winter approaches, COVID cases are expected to rise. A vaccine is also important for children so they do not transmit the virus to more vulnerable people, such as adults at home. Now that the first vaccine has been authorized for ages 5-11, children can safely return to interacting with other children, and parents can have peace of mind that their children are being protected from COVID-19.